Water-soluble diiron hexacarbonyl complex as a CO-RM: controllable CO-releasing, releasing mechanism and biocompatibility†
Abstract
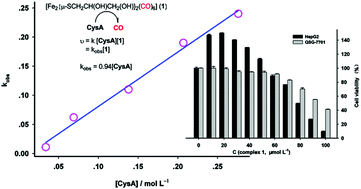
A water soluble diiron hexacarbonyl complex, [Fe2{μ-SCH2CH(OH)CH2(OH)}2(CO)6] (1), was synthesised by reacting thioglycol with Fe3(CO)12 in THF. This diiron complex was employed as carbon monoxide releasing molecule (CO-RM). The CO-releasing was initiated via substitution of the bound CO by cysteamine (CysA, a clinic medicine). Further decomposition of the substituted products led to at least partially the formation of monoiron(II) dicarbonyl species via oxidative process while releasing more CO under inert atmosphere. Three intermediates generated in the CO-releasing process were spectroscopically identified. The kinetics of the decomposition of complex 1 was first-order process for both the complex and CysA, respectively. Under open atmosphere, the CO-releasing mechanism altered due to the involvement of oxygen in the decomposition of complex 1. The system showed minimal cytotoxicity in two selected arbitrarily cell lines, QSG-7701 and HepG2, with IC50 at the scale of 100 μmol L−1.

 Please wait while we load your content...
Please wait while we load your content...