Synthesis of hexa aza cages, SarAr-NCS and AmBaSar and a study of their metal complexation, conjugation to nanomaterials and proteins for application in radioimaging and therapy†
Abstract
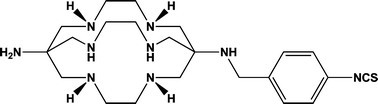
A novel hexa aza cage, N1-(4-isothiocyanatobenzyl)-3,6,10,13,16,19-hexaazabicyclo[6.6.6]icosane-1,8-diamine (SarAr-NCS) was synthesized in good yield and characterized by 1H NMR and electrospray mass spectrometry. A new method for the synthesis of the related N1-(4-carboxybenzyl)-3,6,10,13,16,19-hexaazabicyclo[6.6.6]icosane-1,8-diamine (AmBaSar) using the p-carboxybenzaldehyde is reported. The complexation of Cu2+, Co2+ and Zn2+ by the two ligands over a range of pHs was found to be similar to the parent derivative SarAr. SarAr-NCS was conjugated to both silica particles (≈90 nm diam.) and the model B72.3 murine antibody. The SarAr-NCSN-silica particles were radiolabeled with Cu2+ doped 64Cu and the number of ligands conjugated was calculated to be an average of 7020 ligands per particle. Conjugation of SarAr-NCS to the B72.3 antibody was optimized over a range of conditions. The SarAr-NCSN-B72.3 conjugate was stored in buffer and as a lyophilized powder at 4 °C over 38 days. Its radiolabeling efficiency, stability and immunoreactivity were maintained. The development of a high yielding synthesis of SarAr-NCS should provide an entry point for a wide range of Cu and Zn radiometal PET imaging agents and potentially radiotherapeutic agents with 67Cu.

 Please wait while we load your content...
Please wait while we load your content...