A Co16 cluster and a 1-D Mn chain complex supported by benzohydroxamic acid†
Abstract
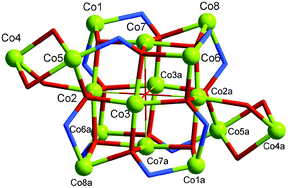
The syntheses, crystal structures and magnetic properties are described for a {Co16} cluster [CoII16O(OH)2(bha)12(PhCO2)4(Phen)2(MeOH)4]·2MeOH (1) and a 1-D MnII chain complex [Mn(Hbha)2]n·(2MeOH)n (2) (H2bha =

 Please wait while we load your content...
Please wait while we load your content...