Molecular and Merrifield supported chiral diamines for enantioselective addition of ZnR2 (R = Me, Et) to ketones†
Abstract
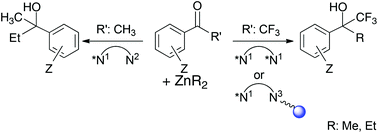
Chiral 1,2-ethylenediamines have been previously reported as active catalysts in the enantioselective addition reactions of ZnR2 to either methyl- or trifluoromethyl-ketones. Subtle changes in the molecular structure of different catalysts are described herein and lead to a dramatic effect in their catalytic activity. From these findings, we demonstrate the selective reactivity of the ligands used in the addition of ZnR2 (R = Me, Et) to methyl- and trifluoromethyl-ketones offering an enantioselective access either to chiral non-fluorinated alcohols or to chiral fluorinated tertiary alcohols. Considering the importance of the chiral trifluoromethyl carbinol fragment in several biologically active compounds, we have extended the scope of the addition reaction of ZnEt2 to several trifluoromethylketones catalyzed by (R,R)-1,2-diphenylethylenediamine derivatives. This work explores a homogeneous approach that provides excellent yields and very high ee and the use of a heterogenized tail-tied ligand affording moderate ee, high yields and allowing an easier handling and recycling.

 Please wait while we load your content...
Please wait while we load your content...