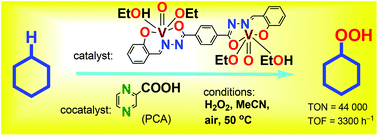
A new binuclear oxovanadium(V) complex [{VO(OEt)(EtOH)}2L] (1) where H4L is bis(2-hydroxybenzylidene)terephthalohydrazide has been synthesized and fully characterized. The combination of 1 with pyrazine-2-carboxylic acid (PCA; a cocatalyst) affords a catalytic system for the efficient oxidation of saturated hydrocarbons, RH, with hydrogen peroxide and air in acetonitrile solution at 50 °C to produce alkyl hydroperoxides, ROOH, as the main primary products. Very high turnover numbers (TONs) have been attained in this reaction: for example, after 2220 min, TON = 44 000 and initial TOF (turnover frequency) = 3300 h−1 per molecule of complex 1. The estimated activation energy of the cyclohexane oxygenation in the presence of 1/PCA is Ea = 16 ± 2 kcal mol−1. This value is identical to that obtained for the cyclohexane oxidation with H2O2 catalyzed by the (n-Bu4N)[VO3]/PCA combination (17 ± 2 kcal mol−1). The dependences of initial oxidation rates W0 on the initial concentrations of all components of the reaction mixture have been determined. Based on these kinetic data and on the regio- and bond-selectivity parameters measured in the oxidation of linear and branched alkanes a mechanism of the oxidation has been proposed which includes the generation of hydroxyl radicals in the crucial stage.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...