The molecular structure of the guanidinate complex {NbBz2(NtBu)[(4-BrC6H4)N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(NiPr)(NHiPr)]}, previously obtained by reaction of [NbBz3(NtBu)] and the corresponding guanidine proligand, has been established by X-ray diffraction. The series of complexes {NbBz2(NtBu)[(Ar)N
C(NiPr)(NHiPr)]}, previously obtained by reaction of [NbBz3(NtBu)] and the corresponding guanidine proligand, has been established by X-ray diffraction. The series of complexes {NbBz2(NtBu)[(Ar)N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(NiPr)(NHiPr)]} (Ar = 4-BrC6H4, 4-tBuC6H4, 4-MeOC6H4) and {[NbBz2(NtBu)]2[(C6H4)(N
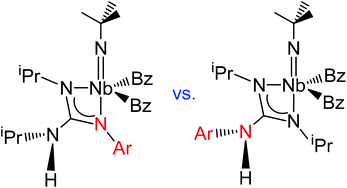
C(NiPr)(NHiPr)]} (Ar = 4-BrC6H4, 4-tBuC6H4, 4-MeOC6H4) and {[NbBz2(NtBu)]2[(C6H4)(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(NiPr)(NHiPr))2]} show a preferred asymmetric coordination of the guanidinate ligand by means of one alkylamino nitrogen and the arylimino nitrogen atom. Computational studies confirm this preference and the results suggest that electronic factors prevail over steric factors. In addition, reaction of complex [NbBz3(NtBu)] with {2-(nbutyl)-1,3-diisopropylguanidine} did not give rise to the regioselective asymmetrical guanidinate. Instead, the complex {NbBz2(NtBu)[(nBu)N
C(NiPr)(NHiPr))2]} show a preferred asymmetric coordination of the guanidinate ligand by means of one alkylamino nitrogen and the arylimino nitrogen atom. Computational studies confirm this preference and the results suggest that electronic factors prevail over steric factors. In addition, reaction of complex [NbBz3(NtBu)] with {2-(nbutyl)-1,3-diisopropylguanidine} did not give rise to the regioselective asymmetrical guanidinate. Instead, the complex {NbBz2(NtBu)[(nBu)N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(NiPr)(NHiPr)]} was obtained as a mixture of three isomers with symmetrical and asymmetrical coordination modes. Finally, the complex [NbBz3(NtBu)] was shown to be a suitable precatalyst for the guanylation reaction of a wide range of amines under mild conditions. Guanidinates are proposed as intermediates in the mechanism of this reaction. The molecular structure of the biguanidine {2,2′-(1,4-phenylene)bis(2′,3-diisopropylguanidine)} was also established by X-ray diffraction studies.
C(NiPr)(NHiPr)]} was obtained as a mixture of three isomers with symmetrical and asymmetrical coordination modes. Finally, the complex [NbBz3(NtBu)] was shown to be a suitable precatalyst for the guanylation reaction of a wide range of amines under mild conditions. Guanidinates are proposed as intermediates in the mechanism of this reaction. The molecular structure of the biguanidine {2,2′-(1,4-phenylene)bis(2′,3-diisopropylguanidine)} was also established by X-ray diffraction studies.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(NiPr)(NHiPr)]}, previously obtained by reaction of [NbBz3(NtBu)] and the corresponding
C(NiPr)(NHiPr)]}, previously obtained by reaction of [NbBz3(NtBu)] and the corresponding ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(NiPr)(NHiPr)]} (Ar = 4-BrC6H4, 4-tBuC6H4, 4-MeOC6H4) and {[NbBz2(NtBu)]2[(C6H4)(N
C(NiPr)(NHiPr)]} (Ar = 4-BrC6H4, 4-tBuC6H4, 4-MeOC6H4) and {[NbBz2(NtBu)]2[(C6H4)(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(NiPr)(NHiPr))2]} show a preferred asymmetric coordination of the guanidinate
C(NiPr)(NHiPr))2]} show a preferred asymmetric coordination of the guanidinate ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(NiPr)(NHiPr)]} was obtained as a mixture of three isomers with symmetrical and asymmetrical coordination modes. Finally, the complex [NbBz3(NtBu)] was shown to be a suitable precatalyst for the guanylation reaction of a wide range of
C(NiPr)(NHiPr)]} was obtained as a mixture of three isomers with symmetrical and asymmetrical coordination modes. Finally, the complex [NbBz3(NtBu)] was shown to be a suitable precatalyst for the guanylation reaction of a wide range of 

 Please wait while we load your content...
Please wait while we load your content...