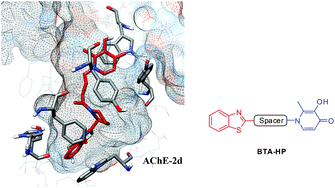
The multifactorial nature of Alzheimer's disease (AD), and the absence of a disease modifying drug, makes the development of new multifunctional drugs an attractive therapeutic strategy. Taking into account the hallmarks of AD patient brains, such as low levels of acetylcholine, misfolding of proteins and associated beta-amyloid (Aβ) aggregation, oxidative stress and metal dyshomeostasis, we have developed a series of compounds that merge three different approaches: metal attenuation, anti-Aβ aggregation and anti-acetylcholinesterase activity. Therefore, 3-hydroxy-4-pyridinone (3,4-HP) and benzothiazole molecular moieties were selected as starting frameworks due to their well known affinity for iron and Aβ peptides, respectively. The linkers between these two main functional groups were selected on the basis of virtual screening, so that the final molecule could further inhibit the acetylcholinesterase, responsible for the cholinergic losses. We describe herein the design and synthesis of the new hybrid compounds, followed by the assessment of solution properties, namely iron chelation and anti-oxidant capacity. The compounds were bioassayed for their capacity to inhibit AChE, as well as self- and Zn mediated-Aβ1–42 aggregation. Finally, we assessed their effects on the viability of neuronal cells stressed with Aβ42.

 Please wait while we load your content...
Please wait while we load your content...