Metal complexes with functionalised 2,2′-dipicolylamine ligand containing an antioxidant 2,6-di-tert-butylphenol moiety: synthesis and biological studies†
Abstract
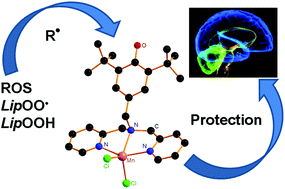
A series of Zn, Mn, Fe, Co, and Ni complexes ([MX2L], X = Cl, OAc) of the novel di-(2-picolyl)amine ligand L with an antioxidant 2,6-di-tert-butylphenol pendant were synthesized and characterized by elemental analysis, IR, multinuclear NMR spectroscopy, MALDI-TOF mass spectrometry and the molecular structures of [ZnCl2L] and [MnCl2L] were established by X-ray crystallography. The chemical oxidation of complexes with a 2,6-di-tert-butylphenol fragment to the phenoxyl radicals was studied by means of ESR method. The antioxidant radical scavenging activity of the complexes was measured spectrophotometrically using a DPPH-test and linoleic acid peroxidation. The electron transfer reactions were examined in CUPRAC tests and as the inhibition of an enzymatic reaction involving the generation of a reactive oxygen species (superoxide radical-anion) by xanthine oxidase. The lipoxygenase (LOX) inhibition activity of the studied compounds was evaluated. The in vitro biological experiments were performed by using rat brain homogenates. The role of the phenol fragment and metal was found to be essential in antioxidant activity.

 Please wait while we load your content...
Please wait while we load your content...