pH luminescence switch, DNA binding and photocleavage, and cytotoxicity of a dinuclear ruthenium complex†
Abstract
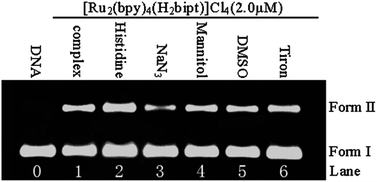
The ground- and excited-state acid–base properties of [Ru2(bpy)4(H2bipt)]Cl41 {bpy = 2,2′-bipyridine, H2bipt = 2,5-bis[1,10]phenanthrolin[4,5-f]-imidazol-2-yl)thiophene} are investigated by emission and UV-visible absorption spectrophotometric pH titrations. The DNA binding properties of 1 are studied by means of DNA viscosity and optical spectroscopic techniques of UV-visible absorption and emission spectral titrations, steady-state emission quenching with ferrocyanide, ethidium bromide competitive binding, and DNA thermal denaturation as well as density functional theoretical calculations. The DNA photocleavage and singlet oxygen generation properties as well as in vitro anticancer activities against five cancer cell lines are studied as well. The results demonstrated that pH-induced luminescence switching, DNA binding, and anticancer properties of 1 are much improved with respect to those of the mononuclear analog [Ru(bpy)2(Htip)]Cl2 {Htip = 2-(thiophen-2-yl)-1H-imidazo[4,5-f][1,10]phenanthroline}.

 Please wait while we load your content...
Please wait while we load your content...