Metal-free dehydrogenation of amine–boranes by an N-heterocyclic carbene†
Abstract
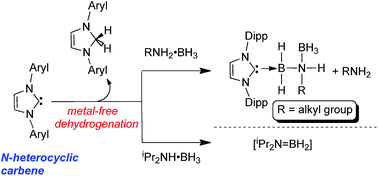
The ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) BH2] and the dihydroaminal IPrH2 as products. Attempts to induce H2 elimination from the arylamine–borane DippNH2·BH3 yielded a reaction mixture containing the known species IPr·BH2NHDipp, IPr·BH2NH(Dipp)–BH3, free DippNH2 and IPrH2. The new hindered aryl-amine borane adduct Ar*NH2·BH3 [Ar* = 2,6-(Ph2CH)2-4-MeC6H2] underwent a reaction with IPr to give IPr·BH3 and free Ar*NH2, consistent with the presence of a weaker N–B dative bond in Ar*NH2·BH3 relative to its less hindered
BH2] and the dihydroaminal IPrH2 as products. Attempts to induce H2 elimination from the arylamine–borane DippNH2·BH3 yielded a reaction mixture containing the known species IPr·BH2NHDipp, IPr·BH2NH(Dipp)–BH3, free DippNH2 and IPrH2. The new hindered aryl-amine borane adduct Ar*NH2·BH3 [Ar* = 2,6-(Ph2CH)2-4-MeC6H2] underwent a reaction with IPr to give IPr·BH3 and free Ar*NH2, consistent with the presence of a weaker N–B dative bond in Ar*NH2·BH3 relative to its less hindered

 Please wait while we load your content...
Please wait while we load your content...