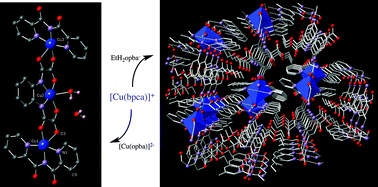
We herein present the synthesis and X-ray structures of five copper(II) complexes of formulae [Cu(bpca)(CF3SO3)(H2O)]·H2O (1), [Cu(bpca)(Phpr)(H2O)]·3/2H2O (2), {[Cu(bpca)]2[Cu(opba)(H2O)]}·H2O (3), {[Cu(bpca)]2(H2opba)}2·6H2O (4) and [Cu(bpca)(EtH2opba)]n (5), where bpca = bis(2-pyridylcarbonyl)amidate, Phpr = 3-phenylpropionate, CF3SO3− = triflate (anion of the trifluoromethanesulphonic acid), H4opba = N,N′-1,2-phenylenebis(oxamic acid), and EtH3opba = monoethyl ester derivative of the H4opba. 1 and 2 are mononuclear copper(II) complexes where the copper atom is five-coordinate in distorted square pyramidal surroundings with a tridentate bpca and a water molecule (1)/carboxylate oxygen (2) building the basal plane and a triflate oxygen (1)/water molecule (2) filling the apical position. 3 is a neutral tricopper(II) complex where the [Cu(opba)(H2O)]2− unit acts as a bis-bidentate ligand toward two peripheral [Cu(bpca)]+ fragments. The three crystallographically independent copper(II) ions in 3 are five-coordinate with two nitrogen and two oxygen atoms (inner copper atom)/three bpca-nitrogen and an oxamate oxygen (outer copper atom) building the basal plane plus a water molecule (inner copper)/an oxamate oxygen (outer copper) in the apical position (inner copper atom) of somewhat distorted square pyramidal surroundings. 4 is a centrosymmetric tetracopper(II) compound where four [Cu(bpca)]+ fragments are assembled by two H2opba2− groups adopting an unusual bidentate/bis-monodentate bridging mode. The two crystallographically independent copper(II) ions in 4 are also five-coordinate having the three bpca-nitrogens in basal positions, the other two sites of the distorted square pyramid being filled by two oxygens of either a bidentate oxamate (at one copper centre) or two bis-monodentate oxamates (at the other copper atom). 5 is a zigzag chain of [Cu(bpca)(H2O)]+ units which are connected through the EtH2opba− ligand adopting a bidentate/monodentate bridging mode across the monodeprotonated oxamate group. Each copper(II) ion in 5 is six-coordinate in an elongated octahedral CuN3O3 chromophore. The magnetic properties of 3–5 were investigated in the temperature range 1.9–300 K. 3 exhibits an intermediate intramolecular antiferromagnetic interaction [J = −65.8(2) cm−1 with the Hamiltonian H = −J(SCu1·SCu2 + SCu2·SCu3)] which leads to a low-lying spin doublet at low temperatures. A weak antiferromagnetic coupling between the inner copper(II) ions occurs in 4 [J = −2.36(2) cm−1, H = −JS1·S2)] and a very small intrachain antiferromagnetic interaction is observed in 5 [J = −0.17(1) cm−1 with H = −J∑iSi·Si+1]. These values are analyzed by means of simple orbital symmetry considerations and compared with those previously reported for parent systems.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...