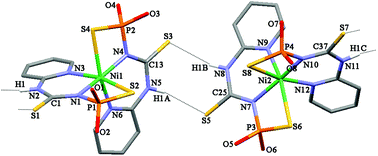
Reaction of the deprotonated N-thiophosphorylated thiourea 2-PyNHC(S)NHP(S)(OiPr)2 (HL) with NiCl2 leads to the complex [Ni{2-PyNHC(S)NP(S)(OiPr)2}2] ([NiL22]) with unprecedented 1,5,7-N,N′,S-coordination of the ligand. Recrystallization of [NiL2] from a mixture of CH2Cl2–n-hexane or acetone–n-hexane leads to [Ni(L-1,5,7-N,N′,S)2]·CH2Cl2 and [Ni(L-1,5,7-N,N′,S)2], respectively. The latter complex, in turn, shows a temperature-induced polymorphism. [NiL2] in solution shows a paramagnetic distorted octahedral structure where the metal center is coordinated through the nitrogen atoms of the phosphorylamide and pyridyl group functions, and oxygen atoms of the phosphorylamide unit. Furthermore, in the solid state at low temperature, [Ni(L-1,5,7-N,N′,S)2] is shown from high-frequency EPR measurements to possess an S = 1 ground state with large anisotropy.

 Please wait while we load your content...
Please wait while we load your content...