Kinetics and mechanism of the manganese(ii) catalysed Calmagite dye oxidation using in situ generated hydrogen peroxide†
Abstract
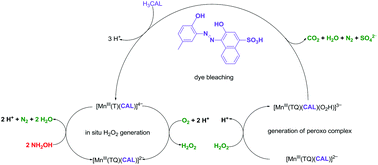
The kinetics and mechanism for the bleaching of Calmagite (H3CAL, 3-hydroxy-4-(2-hydroxy-5-methylphenylazo)naphthalene-1-sulfonic acid) in aqueous solution at pH 8.00 and 23 ± 1 °C using in situ generated H2O2 is described. Complete mineralisation of H3CAL results with turnover frequencies (TOF = moles of H3CAL bleached per mole of manganese per hour) of 40 h−1. The monohydroxy azo dyes Me–H2CAL, Orange G and Orange II are not bleached which indicates that a requirement of dye bleaching is the coordination of the dye to the Mn centre. Spectroscopic studies show the formation of Mn(CAL)2 and Mn(CAL) species but in the presence of Tiron (1,2-dihydroxybenzene-3,5-disulfonate, disodium salt, monohydrate, Na2TH2·H2O), [Mn(CAL)(T)] is formed. It is proposed that a Mn(III)–hydroperoxide species is generated, [Mn(O2H)(CAL)(TQ)] from the in situ generated H2O2, where TQ represents the o-quinone form of Tiron, and this is the active species in the bleaching of coordinated CAL; the formation of this hydroperoxide species is supported by UV/VIS and ESI-MS data. The formation of a Mn(III) species is supported by EPR studies which also show some evidence for the presence of a labile d5 Mn(II) species in the presence of the reducing substrate hydroxylamine (NH2OH). This would enable rapid ligand exchange for both in situ H2O2 generation and dye bleaching to occur; there is no evidence for the presence of MnIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species. The virtue of low local concentrations of in situ generated H2O2 is shown to be important in preventing over oxidation of the catalyst and thus contributing to a robust catalytic system.
O species. The virtue of low local concentrations of in situ generated H2O2 is shown to be important in preventing over oxidation of the catalyst and thus contributing to a robust catalytic system.

 Please wait while we load your content...
Please wait while we load your content...