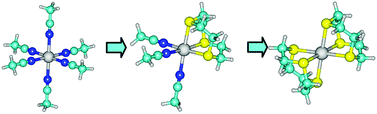
Thermodynamic parameters of complex formation between d10 metal ions, such as Zn2+, Cd2+, Hg2+ and Ag+, and the macrocyclic thioether 1,4,7-trithiacyclononane ([9]AneS3) or the monodentate diethylsulfide (Et2S), in acetonitrile (AN) at 298.15 K, were studied by a systematic methodology including potentiometry, calorimetry and polarography. [9]AneS3 is able to form complexes with all the target cations, Et2S only reacts with Hg2+ and Ag+. Mononuclear MLj (j = 1, 2) complexes are formed with all the metal ions investigated, where the affinity order is Hg2+ > Ag+ > Cd2+ ≈ Zn2+ when L = [9]AneS3 and Hg2+ > Ag+ when L = Et2S. Enthalpy and entropy values are generally negative, as a consequence of both metal ion interactions with neutral ligands, the reagents’ loss of degrees of freedom and the release of solvating molecules. DFT calculations on the complexes formed with [9]AneS3 in vacuum and in AN are also carried out, to correlate experimental and theoretical thermodynamic values and to highlight the interplay between the direct metal–thioether interaction and the solvation effects. Trends obtained for the stability constants and enthalpies of the 1 : 1 and 1 : 2 complexes in solvent well reproduce the experimental ones for all the divalent metal ion complexes with [9]AneS3 and indicate the release of 3 AN molecules in the formation of each consecutive octahedral complex. In addition, calculated and experimental values for Ag+ complex formation in solution suggest that in AgL2 species [9]AneS3 ligands are not both tridentate.

 Please wait while we load your content...
Please wait while we load your content...