Isospecific propylene polymerization with in situ generated bis(phenoxy-amine)zirconium and hafnium single site catalysts†
Abstract
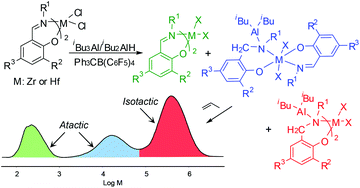
Bis(phenoxy-imine) Zr and Hf complexes were activated with iBu3Al or iBu2AlH in conjunction with Ph3CB(C6F5)4 and tested as
- This article is part of the themed collection: Advances in metal-catalysed polymerisation and related transformations

 Please wait while we load your content...
Please wait while we load your content...