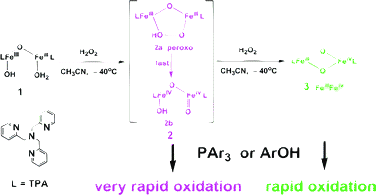
Two intermediates (2 and 3) are formed consecutively in the reaction of a diiron(III) complex [FeIII2(μ-O)(OH)(H2O)(TPA)2](ClO4)3 (TPA = tris(2-pyridylmethyl)amine, tris(picolyl)amine) with H2O2 in CH3CN at −40 °C. Low-temperature stopped-flow studies showed that both species are kinetically competent in oxidation of phosphines and phenols. The first intermediate (2) reacts with substrates very rapidly (second-order rate constants reach 105–106 M−1 s−1 for substituted triarylphosphines and 103–105 M−1 s−1 for substituted phenols), in keeping with a diiron(IV)–oxo formulation. The second intermediate (3), a mixed-valent Fe(III)Fe(IV) species, is more stable than 2, and reacts with substrates more slowly (second-order rate constants range from 150 to 550 M−1 s−1 for triaryl phosphine oxidation, and from 18 to 790 M−1 s−1 for phenol oxidation). Reaction rates increase with increasing electron donating abilities of substituents, indicating that both 2 and 3 act as electrophilic oxidants.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...