Dinuclear aluminum complexes supported by amino- or imino-phenolate ligands: synthesis, structures, and ring-opening polymerization catalysis of rac-lactide†
Abstract
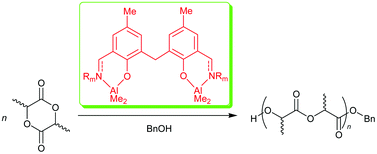
Two series of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NR)-5-MeC6H2]2CH2 (10: R = 2,6-Pri2C6H3; 11: R = p-MeC6H4; 12: R = p-ClC6H4; 13: R = p-MeOC6H4; 14: R = But) were prepared. These compounds reacted with
NR)-5-MeC6H2]2CH2 (10: R = 2,6-Pri2C6H3; 11: R = p-MeC6H4; 12: R = p-ClC6H4; 13: R = p-MeOC6H4; 14: R = But) were prepared. These compounds reacted with ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NR)-5-MeC6H2}]2CH2 (15: R = 2,6-Pri2C6H3; 16: R = p-MeC6H4; 17: R = p-ClC6H4; 18: R = p-MeOC6H4; 19: R = But). All the compounds were characterized by 1H and
NR)-5-MeC6H2}]2CH2 (15: R = 2,6-Pri2C6H3; 16: R = p-MeC6H4; 17: R = p-ClC6H4; 18: R = p-MeOC6H4; 19: R = But). All the compounds were characterized by 1H and

 Please wait while we load your content...
Please wait while we load your content...