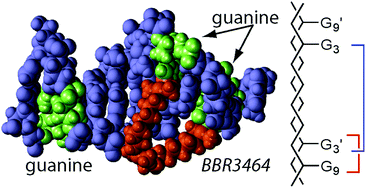
2D [1H, 15N] HSQC NMR spectroscopy has been used to monitor the reaction of fully 15N-labelled [{trans-PtCl(NH3)2}2(μ-trans-Pt(NH3)2{NH2(CH2)6NH2}2)]4+ (Triplatin, BBR3464 or 1,0,1/t,t,t (15N-1)) with the self-complementary 10-mer DNA duplex 5′-{d(ACGTATACGT)2} (duplex I) at pH 5.4 and 298 K. Initial electrostatic interactions were observed in the minor groove of the duplex, followed directly by aquation to form the monoaqua monochloro species. There was evidence for two discrete monofunctional adducts, through covalent binding at the guanine N7 sites, and one had distinctly different 1H/15N chemical shifts to those observed previously in analogous reactions. Bifunctional adduct formation followed by binding at a second guanine N7 site with evidence for both the 3′–3′ 1,2-GG and 5′–5′ 1,6-GG interstrand cross-links in a ratio of 2 : 1. The results show that cross-link preference is kinetically controlled and will depend critically on the reaction conditions, explaining why in a previous reaction of 1 with duplex I the major adduct isolated by HPLC had two simultaneous 3′–3′ 1,2-interstrand cross-links.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...