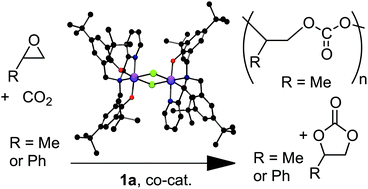
Reaction of CO2 with propylene oxide and styrene oxide catalyzed by a chromium(iii) amine-bis(phenolate) complex†
Abstract
A diamine-bis(phenolate) chromium(III) complex, {CrCl[O2NN′]BuBu}2 catalyzes the copolymerization of
- This article is part of the themed collection: Advances in metal-catalysed polymerisation and related transformations

 Please wait while we load your content...
Please wait while we load your content...