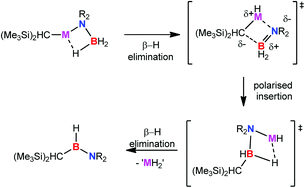
Reactions of equimolar quantities of secondary amine boranes, R2NH·BH3, with the homoleptic group 2 alkyl compounds [M{CH(SiMe3)2}2(THF)2] (M = Mg, Ca, Sr) provide the alkyl group 2 amido borane derivatives [M{CH(SiMe3)2}{NR2BH3}(THF)]2. While the strontium derivatives of reactions with dimethylamine and pyrrolidine borane are stable and isolable compounds, the analogous magnesium and calcium compounds are found to be unstable at room temperature. Studies of the thermolysis of the alkylstrontium derivatives have allowed this instability to be rationalised as a result of β-hydride elimination, the facility of which varies with changing M2+ charge density, to form the products of M–C insertion of H2B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NR2. Subsequent to this process, alkylaminoboranes, [HB(NR2){CH(SiMe3)2}], are observed to form through a further suggested β-hydride elimination reaction. This chemistry is also extended to the reaction of the primary amine borane tBuNH2·BH3 with [Sr{CH(SiMe3)2}2(THF)2]. In this case the crystal structure of a heteroleptic species, which may be considered as a tetrameric aggregate of two [Sr{CH(SiMe3)2}{(NHtBu)BH3}2] anions and two cationic [Sr{(NHtBu)(BH3)}(THF)2] components, has been determined. Kinetic studies of the reactions of [M{CH(SiMe3)2}2(THF)2] (M = Mg, Ca, Sr) with dimethylamine borane have also been undertaken and describe a complex mechanism in which the barriers to formation of the various intermediate species are a consequence of M2+ radius and resultant charge density as well as the steric demands of the coordinated amidoborane ligands.
NR2. Subsequent to this process, alkylaminoboranes, [HB(NR2){CH(SiMe3)2}], are observed to form through a further suggested β-hydride elimination reaction. This chemistry is also extended to the reaction of the primary amine borane tBuNH2·BH3 with [Sr{CH(SiMe3)2}2(THF)2]. In this case the crystal structure of a heteroleptic species, which may be considered as a tetrameric aggregate of two [Sr{CH(SiMe3)2}{(NHtBu)BH3}2] anions and two cationic [Sr{(NHtBu)(BH3)}(THF)2] components, has been determined. Kinetic studies of the reactions of [M{CH(SiMe3)2}2(THF)2] (M = Mg, Ca, Sr) with dimethylamine borane have also been undertaken and describe a complex mechanism in which the barriers to formation of the various intermediate species are a consequence of M2+ radius and resultant charge density as well as the steric demands of the coordinated amidoborane ligands.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NR2. Subsequent to this process, alkylaminoboranes, [HB(NR2){CH(SiMe3)2}], are observed to form through a further suggested β-hydride
NR2. Subsequent to this process, alkylaminoboranes, [HB(NR2){CH(SiMe3)2}], are observed to form through a further suggested β-hydride 

 Please wait while we load your content...
Please wait while we load your content...