Synthesis, structure, magnetic properties and biological activity of supramolecular copper(ii) and nickel(ii) complexes with a Schiff base ligand derived from vitamin B6†
Abstract
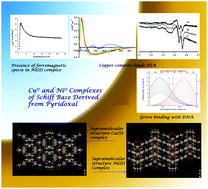
Three new complexes of CuII and NiII, [CuII(H2pydmedpt)]2+·2Cl− (1), [NiII(H2pydmedpt)]2+·2Cl− (2) and [NiII(pydmedpt)(OH)]−·K+ (3) of the Schiff base ligand [H2pydmedpt]2+·2Cl− were synthesized by the in situ reaction of pyridoxal (pyd), a vitamer of vitamin B6, N,N-bis[3-aminopropyl]methylamine (medpt) and copper(II) acetate or nickel(II) acetate, respectively. The molecular structures of 1 and 2 were determined by single crystal X-ray diffraction studies. The structure of 3 in the solid state was inferred by elemental analysis, diffuse reflectance spectrum, variable temperature magnetic moment studies and DFT calculations. The binding of the Schiff base ligand to the metal centers involves two phenolato oxygens, two imine nitrogens and one amine nitrogen. The coordination geometry around Cu in 1 is distorted square pyramidal and that around the Ni atom in 2 is intermediate between square-pyramidal and trigonal-bipyramidal. In the crystals the compounds form supramolecular one dimensional chain structures stabilized by hydrogen bonding and π–π stacking interactions. Variable temperature magnetic moment data of 2 indicate the presence of a momomeric high spin NiII centre in the complex. The solid state diffuse reflectance spectrum, conductance and elemental analysis suggest that 3 is a NiII complex with a tetragonally distorted octahedral field, the sixth position being occupied by the oxygen atom of a hydroxyl group. The variable temperature magnetic moment of 3 indicates the presence of a ferromagnetic dinuclear species (29.2%) along with the major monomeric species, the intra-dimer exchange term J value being 14.3 cm−1. The competitive binding of 1 and 2 with DNA was studied in the concentration range 40 to 400 μM, the apparent binding constants being K = 2.9 × 103 and 6.7 × 103 M−1, respectively. Human Serum Albumin (HSA) binding studies were carried out at concentrations of 800–1000 μM and 400–500 μM for the complexes and HSA, respectively, in PBS buffer at pH 7.4. Complex 1 binds to HSA, while no binding is observed in case of 2, instead, the complex hydrolyses under the experimental conditions used and the resulting Ni2+ ions bind with HSA.

 Please wait while we load your content...
Please wait while we load your content...