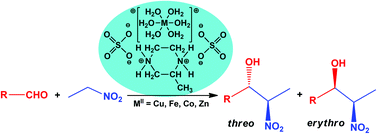
A series of double dihydromethylpiperazinediium metallic sulfates 1–7 [H2mpz]MII(SO4)2·nH2O (mpz = 2-methylpiperazine, C5H12N2) are prepared by slow evaporation using a racemic (R/S)-mpz (for 1, 2) or enantiopure R-mpz (for 3), S-mpz (for 4–7) and sulfates of CuII (for 5), FeII (for 1, 4), CoII (for 7) and ZnII (for 2, 3, 6), and characterized by infrared spectroscopy, elemental analysis and single crystal X-ray diffraction. The [MII(H2O)6]2+, [(R/S)-H2mpz]2+, [(R)-H2mpz]2+ or [(S)-H2mpz]2+ cations and 2SO42− anions are linked together via two types of hydrogen bonds, Ow–Hw⋯O and N–H⋯O, leading to supramolecular arrangements. The use of the racemic 2-mpz provides alternatives in crystallization: a centrosymmetric structure where the enantiomers are related by an appropriate crystallographic symmetry operation or one where the enantiomers occupy the same crystallographic position, generating disorder. Compounds 1–7 act as diastereoselective catalysts for the nitroaldol (Henry) reaction. The diastereoselectivity can be regulated from exclusive threo to exclusive erythro isomer preparation with typical yields of 50–99%, depending on the catalyst and the substrate used.

 Please wait while we load your content...
Please wait while we load your content...