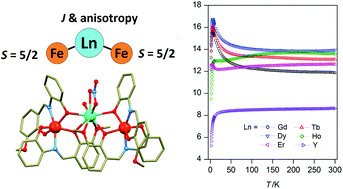
A series of the trinuclear [{Fe(3MeO-L)2}2{μ6-Ln(η2-NO3)(H2O)}]·nH2O, (Ln = Gd (2a), Tb (2b), Dy (2c), Ho (2d), Er (2e), Y (2f), H2-3MeO-L = 2-hydroxy-3-methoxy-phenylsalicylaldimine) complexes were prepared and thoroughly characterized. The crystal structure of 2b was determined and it revealed that the heterotrinuclear complex consists of two anionic [Fe(3MeO-L)2]− subunits coordinated to the [Tb(H2O)(η2-NO3)]2+ bridging moiety through the phenolato and methoxy oxygen atoms. The angular distortion within the coordination polyhedron of the [Fe(3MeO-L)2]− subunits grows significantly upon coordination to the Ln atom of the bridging moiety, which consequently induces an increase in the parameter of the axial magnetic anisotropy. This conclusion is obvious from the comparison and analysis of the structural (XRD) and magnetic data of the yttrium trimer 2f and the precursor complex (Pr3NH)[Fe(3MeO-L)2] (1, Pr3NH = the tripropylammonium cation), where DFe(1) = +0.80 cm−1 and DFe(2f) = +1.64 cm−1. Furthermore, a weak antiferromagnetic interaction between the Fe(III) centres was found in 2f (JFeFe = −0.26 cm−1). The magnetic parameters of 2f were used in the fitting of the magnetic properties of 2a as constraints. The ferromagnetic nature of the Fe–Gd interaction in 2a was confirmed, with JGdFe = +1.40 cm−1, DGd = −0.26 cm−1. Moreover, in the case of the Tb (2b) and Dy (2c) compounds, a slow relaxation of the magnetization at low temperature (below 1.9 K) was observed upon the dehydration of the parent compounds.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...