Incorporation of triazole into a quinoline-rhodamine conjugate imparts iron(iii) selective complexation permitting detection at nanomolar levels†
Abstract
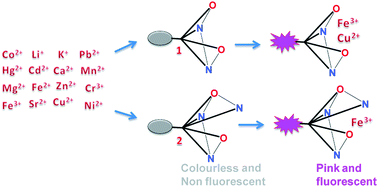
Two new rhodamine based probes 1 and 2 for the detection of Fe3+ were synthesized and their selectivity towards Fe3+ ions in the presence of other competitive metal ions tested. The probe 1 formed a coloured complex with Fe3+ as well as Cu2+ ions and revealed the lack of adequate number of coordination sites for selective complexation with Fe3+. Incorporation of a triazole unit to the chelating moiety of 1 resulted in the probe 2, that displayed Fe3+ selective complex formation even in the presence of other competitive metal ions like Li+, Na+, K+, Cu2+, Mg2+, Ca2+, Sr2+, Cr3+, Mn2+, Fe2+, Co2+, Ni2+, Zn2+, Cd2+, Hg2+ and Pb2+. The observed limit of detection of Fe3+ ions (5 × 10−8 M) confirmed the very high sensitivity of 2. The excellent stability of 2 in physiological pH conditions, non-interference of

 Please wait while we load your content...
Please wait while we load your content...