Synthetic routes to methyl(aryl)alkynylpalladium(IV) motifs are presented, together with studies of selectivity in carbon–carbon coupling by reductive elimination from PdIV centres. The iodonium reagents IPh(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR)(OTf) (R = SiMe3, But, OTf = O3SCF3) oxidise PdIIMe(p-Tol)(L2) (1–3) [L2 = 1,2-bis(dimethylphosphino)ethane (dmpe) (1), 2,2′-bipyridine (bpy) (2), 1,10-phenanthroline (phen) (3)] in acetone-d6 or toluene-d9 at −80 °C to form complexes PdIV(OTf)Me(p-Tol)(C
CR)(OTf) (R = SiMe3, But, OTf = O3SCF3) oxidise PdIIMe(p-Tol)(L2) (1–3) [L2 = 1,2-bis(dimethylphosphino)ethane (dmpe) (1), 2,2′-bipyridine (bpy) (2), 1,10-phenanthroline (phen) (3)] in acetone-d6 or toluene-d9 at −80 °C to form complexes PdIV(OTf)Me(p-Tol)(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR)(L2) [R = SiMe3, L2 = dmpe (4), bpy (5), phen (6); R = But, L2 = dmpe (7), bpy (8), phen (9)] which reductively eliminate predominantly (>90%) p-Tol-C
CR)(L2) [R = SiMe3, L2 = dmpe (4), bpy (5), phen (6); R = But, L2 = dmpe (7), bpy (8), phen (9)] which reductively eliminate predominantly (>90%) p-Tol-C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR above ∼−50 °C. NMR spectra show that isomeric mixtures are present for the PdIV complexes: three for dmpe complexes (4, 7), and two for bpy and phen complexes (5, 6, 8, 9), with reversible reduction in the number of isomers to two occurring between −80 °C and −60 °C observed for the dmpe complex 4 in toluene-d8. Kinetic data for reductive elimination from PdIV(OTf)Me(p-Tol)(C
CR above ∼−50 °C. NMR spectra show that isomeric mixtures are present for the PdIV complexes: three for dmpe complexes (4, 7), and two for bpy and phen complexes (5, 6, 8, 9), with reversible reduction in the number of isomers to two occurring between −80 °C and −60 °C observed for the dmpe complex 4 in toluene-d8. Kinetic data for reductive elimination from PdIV(OTf)Me(p-Tol)(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CSiMe3)(dmpe) (4) yield similar activation parameters in acetone-d6 (66 ± 2 kJ mol−1, ΔH‡ 64 ± 2 kJ mol−1, ΔS‡ −67 ± 2 J K−1 mol−1) and toluene-d8 (Ea 68 ± 3 kJ mol−1, ΔH‡ 66 ± 3 kJ mol−1, ΔS‡ −74 ± 3 J K−1 mol−1). The reaction rate in acetone-d6 is unaffected by addition of sodium triflate, indicative of reductive elimination without prior dissociation of triflate. DFT computational studies at the B97-D level show that the energy difference between the three isomers of 4 is small (12.6 kJ mol−1), and is similar to the energy difference encompassing the six potential transition state structures from these isomers leading to three feasible C–C coupling products (13.0 kJ mol−1). The calculations are supportive of reductive elimination occurring directly from two of the three NMR observed isomers of 4, involving lower activation energies to form p-TolC
CSiMe3)(dmpe) (4) yield similar activation parameters in acetone-d6 (66 ± 2 kJ mol−1, ΔH‡ 64 ± 2 kJ mol−1, ΔS‡ −67 ± 2 J K−1 mol−1) and toluene-d8 (Ea 68 ± 3 kJ mol−1, ΔH‡ 66 ± 3 kJ mol−1, ΔS‡ −74 ± 3 J K−1 mol−1). The reaction rate in acetone-d6 is unaffected by addition of sodium triflate, indicative of reductive elimination without prior dissociation of triflate. DFT computational studies at the B97-D level show that the energy difference between the three isomers of 4 is small (12.6 kJ mol−1), and is similar to the energy difference encompassing the six potential transition state structures from these isomers leading to three feasible C–C coupling products (13.0 kJ mol−1). The calculations are supportive of reductive elimination occurring directly from two of the three NMR observed isomers of 4, involving lower activation energies to form p-TolC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CSiMe3 and earlier transition states than for other products, and involving coupling of carbon atoms with higher s character of σ-bonds (sp2 for p-Tol, sp for C
CSiMe3 and earlier transition states than for other products, and involving coupling of carbon atoms with higher s character of σ-bonds (sp2 for p-Tol, sp for C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–SiMe3) to form the product with the strongest C–C bond energy of the potential coupling products. Reductive elimination occurs predominantly from the isomer with Me3SiC
C–SiMe3) to form the product with the strongest C–C bond energy of the potential coupling products. Reductive elimination occurs predominantly from the isomer with Me3SiC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C trans to OTf. Crystal structure analyses are presented for PdIIMe(p-Tol)(dmpe) (1), PdIIMe(p-Tol)(bpy) (2), and the acetonyl complex PdIIMe(CH2COMe)(bpy) (11).
C trans to OTf. Crystal structure analyses are presented for PdIIMe(p-Tol)(dmpe) (1), PdIIMe(p-Tol)(bpy) (2), and the acetonyl complex PdIIMe(CH2COMe)(bpy) (11).
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR)(OTf) (R = SiMe3, But, OTf = O3SCF3) oxidise PdIIMe(p-Tol)(L2) (1–3) [L2 =
CR)(OTf) (R = SiMe3, But, OTf = O3SCF3) oxidise PdIIMe(p-Tol)(L2) (1–3) [L2 = ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR)(L2) [R = SiMe3, L2 = dmpe (4),
CR)(L2) [R = SiMe3, L2 = dmpe (4), ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR above ∼−50 °C.
CR above ∼−50 °C. ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CSiMe3)(dmpe) (4) yield similar
CSiMe3)(dmpe) (4) yield similar ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CSiMe3 and earlier transition states than for other products, and involving coupling of carbon atoms with higher s character of σ-bonds (sp2 for p-Tol, sp for C
CSiMe3 and earlier transition states than for other products, and involving coupling of carbon atoms with higher s character of σ-bonds (sp2 for p-Tol, sp for C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–SiMe3) to form the product with the strongest C–C bond energy of the potential coupling products. Reductive elimination occurs predominantly from the isomer with Me3SiC
C–SiMe3) to form the product with the strongest C–C bond energy of the potential coupling products. Reductive elimination occurs predominantly from the isomer with Me3SiC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C trans to OTf. Crystal structure analyses are presented for PdIIMe(p-Tol)(dmpe) (1), PdIIMe(p-Tol)(bpy) (2), and the
C trans to OTf. Crystal structure analyses are presented for PdIIMe(p-Tol)(dmpe) (1), PdIIMe(p-Tol)(bpy) (2), and the 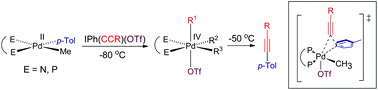

 Please wait while we load your content...
Please wait while we load your content...