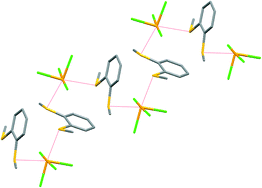
TeF4 reacts with OPR3 (R = Me or Ph) in anhydrous CH2Cl2 to give the colourless, square based pyramidal 1 : 1 complexes [TeF4(OPR3)] only, in which the OPR3 is coordinated basally in the solid state, (R = Me: d(Te–O) = 2.122(2) Å; R = Ph: d(Te–O) = 2.1849(14) Å). Variable temperature 19F{1H}, 31P{1H} and 125Te{1H} NMR spectroscopic studies strongly suggest this is the low temperature structure in solution, although the systems are dynamic. The much softer donor ligands SMe2 and SeMe2 show a lower affinity for TeF4, although unstable, yellow products with spectroscopic features consistent with [TeF4(EMe2)] are obtained by the reaction of TeF4 in neat SMe2 or via reaction in CH2Cl2 with SeMe2. TeX4 (X = F, Cl or Br) causes oxidation and halogenation of TeMe2 to form X2TeMe2. The Br2TeMe2 hydrolyses in trace moisture to form [BrMe2Te–O–TeMe2Br], the crystal structure of which has been determined. TeX4 (X = Cl or Br) react with the selenoethers SeMe2, MeSe(CH2)3SeMe or o-C6H4(SeMe)2 (X = Cl) in anhydrous CH2Cl2 to give the distorted octahedral monomers trans-[TeX4(SeMe2)2], cis-[TeX4{MeSe(CH2)3SeMe}] and cis-[TeCl4{o-C6H4(SeMe)2}], which have been characterised by IR, Raman and multinuclear NMR (1H, 77Se{1H} and 125Te{1H}) spectroscopy, and via X-ray structure determinations of representative examples. Tetrahydrothiophene (tht) can form both 1 : 1 and 1 : 2 Te : L complexes. For X = Br, the former has been shown to be a Br-bridged dimer, [Br3(tht)Te(μ-Br)2TeBr3(tht)], by crystallography with the tht ligands anti, whereas the latter are trans-octahedral monomers. Like its selenoether analogue, MeS(CH2)3SMe forms distorted octahedral cis-chelates, [TeX4{MeS(CH2)3SMe}], whereas the more rigid o-C6H4(SMe)2 unexpectedly forms a zig-zag chain polymer in the solid state, [TeCl4{o-C6H4(SMe)2}]n, in which the dithioether adopts an extremely unusual bridging mode. This is in contrast to the chelating monomer, cis-[TeCl4{o-C6H4(SeMe)2}], formed with the analogous selenoether and may be attributed to small differences in the ligand chelate bite angles. The wider bite angle xylyl-linked bidentates, o-C6H4(CH2EMe2)2 behave differently; the thioether forms cis-chelated [TeX4{o-C6H4(CH2SMe)2}] confirmed crystallographically, whereas the selenoether undergoes C–Se cleavage and rearrangement on treatment with TeX4, forming the cyclic selenonium salts, [C9H11Se]2[TeX6]. The tetrathiamacrocycle, [14]aneS4 (1,4,8,11-tetrathiacyclotetradecane), does not react cleanly with TeCl4, but forms the very poorly soluble [TeCl4([14]aneS4)]n, shown by crystallography to be a zig-zag polymer with exo-coordinated [14]aneS4 units linked via alternate S atoms to a cis-TeCl4 unit. Trends in the 125Te{1H} NMR shifts for this series of Te(IV) halides chalcogenoether complexes are discussed.

 Please wait while we load your content...
Please wait while we load your content...