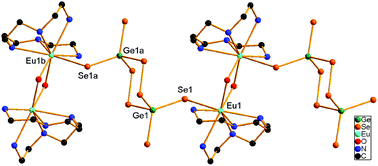
Solvothermal reactions of elements Ge and Se with Ln2O3 in a pentadentate polyamine, tetraethylenepentamine (tepa), produced novel neutral lanthanide–selenidogermanate polymers [{Ln(tepa)(μ-OH)}2(μ-Ge2Se8)]n (Ln = Eu 1, Gd 2, Dy 3). The reaction with Dy2O3 in ethylenediamine (en) afforded an ionic selenidogermanate [{Dy(en)3}2(μ-OH)2]Ge2Se6 (4). In compounds 1–3, the Ln3+ ions are coordinated by a tepa and two OH− ligands to form binuclear [{Ln(tepa)}2(μ-OH)2]4+ fragments. Two GeSe4 tetrahedra are linked through two Se–Se bonds to form a novel [Ge2Se8]4− unit containing a six-membered Ge2Se4 ring in the chair conformation. The [Ge2Se8]4− unit acts as a bridging ligand via the trans terminal Se atoms to interlink the [{Ln(tepa)}2(μ-OH)2]4+ fragments into one-dimensional polymers [{Ln(tepa)(μ-OH)}2(μ-Ge2Se8)]n. Compounds 1–3 are the first examples of solvothermally prepared lanthanide complexes with a selenidogermanate anion as a ligand. The [Ge2Se6]4− anion in 4 is composed of two GeSe4 tetrahedra sharing a common edge, and is charge compensated by a [{Dy(en)5}2(μ-OH)2]4+ complex cation. The formation of the [Ge2Se8]4− and [Ge2Se6]4− anions and their behavior towards lanthanide ions in 1–4 show the significant influence of ethylene polyamines on the solvothermal synthesis of Ln selenidogermanates.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...