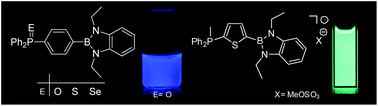
A series of 1,4-phenylenes X-C6H4-BDB with a 1,3,2-benzodiazaborolyl (BDB) and a phosphorus based end group [X = PPh2 (2), P(O)Ph2 (3), P(S)Ph2 (4), P(Se)Ph2 (5), P(AuCl)Ph2 (6) and P(Me)Ph2 (7)] as well as 2-(2′)thienyl-1,3,2-benzodiazaboroles with a second end group X [X = PPh2 (8), P(S)Ph2 (9), P(Se)Ph2 (10) and P(Me)Ph2 (11)] in the 5′ position were synthesised using established methodologies. Molecular structures of 2–9 and 11 were determined by X-ray diffraction. Compounds 3, 4, 6, 7, 9 and 11 show intense blue luminescence in cyclohexane, toluene, chloroform, dichloromethane and tetrahydrofuran with pronounced solvatochromism. Thereby Stokes shifts in the range of 8950–10 440 cm−1 and quantum yields up to 0.70 were observed in dichloromethane solutions. In contrast to this, for the selenides 5 and 10 quantum yields are small (<0.1). The absorption maxima (298–340 nm) are well reproduced by TD-DFT computations (B3LYB/G-311G(d,p)) and arise from strong HOMO–LUMO transitions. With the exception of 5 and 10 the HOMOs of the molecules under study are mainly located on the benzodiazaborole group. In 5 and 10 the HOMOs are on the selenium atoms. The LUMOs of all new neutral molecules are mainly represented by the phenylene or thiophene bridge. In the phosphonium cations the LUMOs have additional contributions from the phosphonium unit.

 Please wait while we load your content...
Please wait while we load your content...