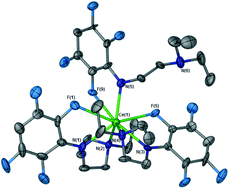
A new class of homoleptic organoamido rare earth complexes [Ln(LMe or LEt)3] (Ln = La, Ce, Nd; LMe/Et = p-HC6F4N(CH2)2NMe2/Et2) exhibiting (Ar)CF–Ln interactions has been isolated from redox–transmetallation/protolysis (RTP) reactions between the free metals, Hg(C6F5)2 and LMe/EtH in tetrahydrofuran, together with low yields of [Ln(LMe)2F]3 (Ln = La, Ce) or [Nd(LEt)2F]2 species, resulting from C–F activation reactions. The structures of the homoleptic complexes have eight-coordinate Ln metals with two tridentate (N,N′,F) amide ligands including (Ar)CF–Ln bonds and either a bidentate (N,F) ligand (Ln = La, Ce, Nd; LEt) or a bidentate (N,N′) ligand (Ln = Nd; LMe), in an unusual case of linkage variation. All (Ar)CF–Ln bond lengths are shorter than or similar to the corresponding Ln–NMe2/Et2 bond lengths. In [Ln(LMe)2F]3 (Ln = La, Ce) complexes, there is a six-membered ring framework with alternating F and Ln atoms and the metal atoms are eight-coordinate with two tridentate (N,N′,F) LMe ligands, whilst [Nd(LEt)2F]2 is a fluoride-bridged dimer.

 Please wait while we load your content...
Please wait while we load your content...