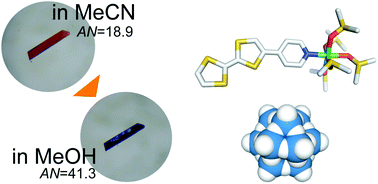
A new salt—[NiII(DMSO)5(TTFPy)]2[α-SiW12O40] (1)—based on polyoxometalates was prepared by coordinating a cationic electron donor of pyridyltetrathiafulvalene (TTFPy) with NiII. Although the TTFPy molecule did not form a salt with the anionic α-[SiWVI12O40]4− because of the weak charge-transfer (CT) interaction, the coordination of Ni with the pyridyl moiety permitted salt formation driven by electrostatic interaction, giving a single crystal of 1. Crystallographic analysis, UV-vis and IR spectroscopy and electrochemical characterization revealed that the fully oxidized α-[SiWVI12O40]4− was crystallized with the neutral TTFPy moiety from the acetonitrile solution because of the low electron-withdrawing ability of α-[SiWVI12O40]4−, forming a brown–orange crystal. The crystal colour quickly turned to black by immersing in methanol, due to CT from TTF moiety to α-[SiWVI12O40]4−, which was caused by the solvent effect. Increase in the solvent acceptor number from 18.9 for acetonitrile to 41.3 for methanol resulted in the enhancement of the electron withdrawing ability of α-[SiWVI12O40]4− by 0.317 V in methanol.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...