Low density ionogels obtained by rapid gellification of tetraethyl orthosilane assisted by ionic liquids
Abstract
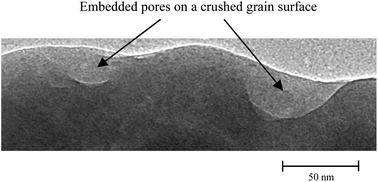
A non-hydrolytic one pot sol–gel method has been used to synthesize mesoporous silica ionogels with the confined ionic liquid (IL) 1-ethyl 3-methyl imidazolium tetra fluoro-borate [EMIM][BF4]. The precursor for obtaining the SiO2 matrix was tetraethyl orthosilicate (TEOS) and formic acid was used as a catalyst. These ionogels have been characterized by density measurements, TEM, BET, DSC, TGA and FTIR. The incorporation of the ionic liquid [EMIM][BF4] enhances the gellification rate which results in the ionogels having very low density (∼0.3 g cm−3). The low density has been explained on the basis of the creation of ‘blind embedded pores’ in the matrix (apart from open pores) due to very rapid gellification (∼1 min). Morphological studies provide experimental evidence for the presence of blind pores/voids inside the ionogel ingots. We have also shown that the IL entrapped in nanopores (∼7–8 nm pore size) of the SiO2 matrix has different physical properties than the bulk IL viz. (a) the phase transition temperatures (Tg, Tc and Tm) of the IL change upon confinement, (b) the thermal stability reduces upon confinement, and (c) the pore wall interaction with the IL results in changes in the C–H vibrations of the imidazolium ring and alkyl chain (the former increasing) which is also indicated in our DFT-calculation.

 Please wait while we load your content...
Please wait while we load your content...