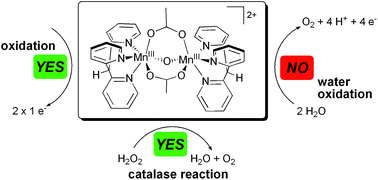
In this work the synthesis of the novel manganese complex [Mn2III,III(tpdm)2(μ-O)(μ-OAc)2]2+ (1) is reported, containing two manganese centres ligated to the unusual, facially coordinating, all-pyridine ligand tpdm (tris(2-pyridyl)methane). The geometric and electronic properties of complex 1 were characterised by X-ray crystallography, vibrational (IR and Raman) and optical spectroscopy (UV/Vis and MCD). Cyclic voltammograms of 1 showed a quasi-reversible oxidation event at 950 mV and an irreversible reduction wave at −250 mV vs. Ag/Ag+. The redox behaviour of the compound was investigated in detail by UV/Vis- and X-band EPR-spectroelectrochemistry. Both electrochemical (+1200 mV) and chemical (tBuOOH) oxidations transform 1 into the singly oxidized di-μ-oxido species [Mn2III,IV(tpdm)2(μ-O)2(μ-OAc)]2+. Further electrochemical oxidation at the same potential results in the removal of a second electron to obtain a Mn2IV,IV-species. The ability of compound 1 to evolve O2 was studied using different reaction agents. While reactions with both hydrogen peroxide and peroxomonosulfate yield O2, homogeneous water-oxidation using CeIV was not observed. Nevertheless, the oxidation reactions of 1 are very interesting model processes for oxidation state (S-state) transitions of the natural manganese water-oxidation catalyst in photosynthesis. However, despite its favourable coordination geometry and multielectron redox chemistry, complex 1 fails to be a catalytically active model for natural water-oxidation.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...