Synthesis, structure and reactivity of 4-phosphanylated 1,3-dialkyl-imidazole-2-thiones†
Abstract
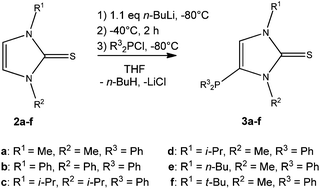
Selective formation of 4-phosphanylated 1,2-dialkyl imidazole-2-thiones 3a–f has been obtained via a lithiation followed by phosphanylation reaction. The reactivity of 3a–f was examined towards

 Please wait while we load your content...
Please wait while we load your content...