pH-dependent assembly of two inorganic–organic hybrid compounds based on octamolybdates: an unusual intercalated layer and a 3D 4-connected framework†
Abstract
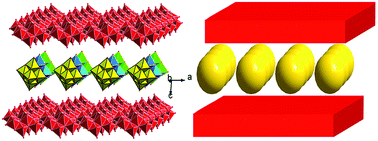
Two novel inorganic–organic hybrid compounds based on octamolybdates, namely, [Cu(H2L)2(γ-Mo8O26)]·(Mo6O19)·2H2O (1) and [Cu(H2L)(γ-Mo8O26)(H2O)2]·5H2O (2), where L = 1,1′-(1,5-pentanediyl)bis[2-(4-pyridyl)benzimidazole], have been successfully synthesized at different pH values under hydrothermal conditions. Compound 1, which is hydrothermally prepared at pH ≈ 3.5, exhibits an entirely new type of intercalated layer. The nanosized hexamolybdate anions as guests are introduced into the layers. When the pH value is adjusted to 2, a structurally-different complex 2 was obtained. Compound 2 shows a unique 3D 4-connected framework constructed by inorganic layers and H2L2+ ligands as bridges. The two compounds were characterized by elemental analyses, IR spectra and TGA. In addition, the electrochemical properties of 1-modified carbon paste electrode (CPE) have also been investigated in 1 M H2SO4 aqueous solution.
- This article is part of the themed collection: Polyoxometalates

 Please wait while we load your content...
Please wait while we load your content...