Synthesis and characterization of benzoxazine-functionalized amine bridged bis(phenolate) lanthanide complexes and their application in the ring-opening polymerization of cyclic esters†
Abstract
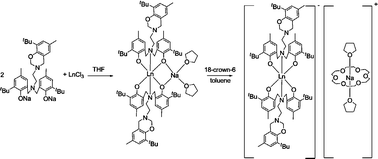
The synthesis and structures of lanthanide complexes supported by ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Y (1), Nd (2), Er (3), Yb (4)). Further treatment of the product with
Y (1), Nd (2), Er (3), Yb (4)). Further treatment of the product with ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Y (5), Yb (6)). The single-crystal structural analyses of 1 and 3–6 revealed that the lanthanide
Y (5), Yb (6)). The single-crystal structural analyses of 1 and 3–6 revealed that the lanthanide

 Please wait while we load your content...
Please wait while we load your content...