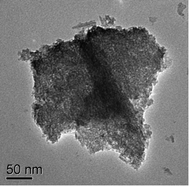
Nano-structured 2-line ferrihydrite was synthesized by a pH-controlled precipitation technique at 90 °C. Chemical, X-ray diffraction (XRD), Fourier transform infrared (FTIR) and Raman analyses confirmed the sample to be 2-line ferrihydrite. The nano nature of the prepared sample was studied by transmission electron microscopy (TEM). The surface area obtained by the Brunauer–Emmett–Teller (BET) method was 175.8 m2 g−1. The nanopowder so obtained was used to study its behaviour for the removal of Pb(II), Cd(II), Cu(II) and Zn(II) from aqueous solutions. The relative importance of experimental parameters such as solution pH, contact time and concentration of adsorbate on the uptake of various cations was evaluated. By increasing the pH from 2.0 to 5.5, adsorption of the four cations increased. The kinetics parameters were compared by fitting the contact time data to both linear as well as non-linear forms of pseudo-second-order models. Linear forms of both Langmuir and Freundlich models fitted the equilibrium data of all the cations except for Pb(II) which was also fitted to the non-linear forms of both the models as it gave a low R2 value of 0.85 for the Langmuir model. High Langmuir monolayer capacities of 366, 250, 62.5 and 500 mg g−1 were obtained for Pb(II), Cd(II), Cu(II) and Zn(II), respectively. Presence of chloride or sulfate had an adverse effect on cation adsorption. The interactive effects on adsorption from solutions containing two, three or four cations were studied. Surprisingly no Cd(II) adsorption was observed in Pb(II)–Cd(II), Pb(II)–Cd(II)–Zn(II) and Pb(II)–Cd(II)–Cu(II)–Zn(II) systems under the studied concentration range. The overall loading capacity of the adsorbent decreased in mixed cation systems. Metal ion loaded adsorbents were characterized by XRD, FTIR and Raman techniques. The high adsorption capability of the 2-lines ferrihydrite makes it a potentially attractive adsorbent for the removal of cations from aqueous solutions.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...