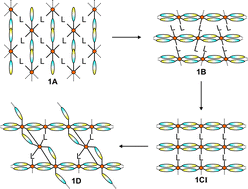
In this contribution several new coordination compounds on the basis of cadmium(II) thio- and selenocyanate with pyrimidine as co-ligand were prepared and investigated for their structural, thermal and spectroscopic properties. The reaction of cadmium(II) thiocyanate with pyrimidine leads to the formation of four compounds, which from a structural point of view are closely related. In the most pyrimidine-rich 1 : 2 compound [Cd(NCS)2(pyrimidine)2]n (1A) (1 : 2 = ratio between metal salt and the co-ligand pyrimidine) the Cd cations are linked by the pyrimidine ligands into layers and are additionally coordinated by two terminal N-bonded anions. In the 2 : 3 compound {[Cd(NCS)2]2(pyrimidine)3}n (1B) the Cd cations are linked into chains by μ-1,3 bridging thiocyanato anions, which are connected into layers by only half of the pyrimidine ligands, whereas the other co-ligands are only terminal coordinated. Further reduction of the pyrimidine content leads to the formation of the 1 : 1 2D compound [Cd(NCS)2(pyrimidine)]n (1CI) in which the terminal N-bonded thiocyanato anions become bridging. Surprisingly, crystallization experiments lead to the formation of an additional pyrimidine-deficient intermediate of composition {[Cd(NCS)2]3(pyrimidine)2}n (1D), in which some of the μ-1,3 coordinated anions transform into μ-1,1,3 bridging thiocyanato anions. Consequently the four structures can be used as snapshots of intermediates on the way to a more condensed thiocyanato coordination network. In contrast, with cadmium selenocyanate only two different compounds were obtained. The 1 : 2 compound [Cd(NCSe)2(pyrimidine)2]n (2A) is not isotypic to 1A and shows a completely different coordination topology whereas the pyrimidine-deficient 1 : 1 compound (2B) shows a more condensed network with μ-1,3 coordinating selenocyanato anions. On heating, the 1 : 2 compound 1A decomposes into Cd(NCS)2via a new polymorphic modification (1CII) as intermediate which is metastable, whereas the 1 : 2 selenocyanato compound 2A transforms into the 1 : 1 compound 2B on heating which cannot be obtained phase pure under these conditions. If faster heating rates are used, there are indications for the formation of a 3 : 2 compound, which is amorphous to X-rays. The results are compared with those obtained for related thio- and selenocyanato coordination polymers with pyridine, pyridazine and pyrazine as co-ligand. Moreover, their impact on the structures and thermal reactivity of analogous paramagnetic compounds is discussed in detail. Based on the structural data of compound 1D the unknown structures of two intermediates were determined, which are formed in the thermal decomposition reaction of the Mn and Fe thiocyanato pyrimidine coordination polymers, reported recently.

 Please wait while we load your content...
Please wait while we load your content...