A study of the step-wise oxidation of a Ni(II) diaminodithiolate complex through the formation of sulfate, the ultimate sulfur oxygenate, is reported. Controlled oxygenations or peroxidations of a neutral, planar, tetracoordinate, low-spin Ni(II) complex of a N2S2-donor ligand, (N,N′-dimethyl-N-N′-bis(2-mecaptoethyl)-1,3-propanediaminato) nickel(II) (1), led to a series of sulfur oxygenates that have been isolated and characterized by ESI-MS and single-crystal X-ray diffraction. A monosulfenate complex (2) was detected by ESI-MS as a product of oxidation with one equivalent of H2O2. However, this complex proved too unstable to isolate. Reaction of the dithiolate (1) with two equivalents of H2O2 or one O2 molecule leads to the formation of a monosulfinate complex (3), which was isolated and fully characterized by crystallography. The oxidation product of the monosulfinate (3) produced with either O2 or H2O2 is an interesting dimeric complex containing both sulfonate and thiolate ligands (4), this complex was fully characterized by crystallography, details of which were reported earlier by us. A disulfonate complex (7) is produced by reaction of 1 in the presence of O2 or by reaction with exactly six equivalents of H2O2. This complex was isolated and also fully characterized by crystallography. Possible intermediates in the conversion of the monosulfinate complex (3) to the disulfonate complex (7) include complexes with mixed sulfonate/sulfenate (5) or sulfonate/sulfinate (6) ligands. Complex 5, a four-oxygen adduct of 1, was not detected, but the sulfonate/sulfinate complex (6) was isolated and characterized. The oxidation chemistry of 1 is very different from that reported for other planar cis-N2S2 Ni(II) complexes including N,N′-dimethyl-N-N′-bis(2-mecaptoethyl)-1,3-ethylenediaminato) nickel(II), (8), and N,N'-bis(mercaptoethyl)-1,5-diazacyclooctane nickel(II). To address the structural aspects of the reactivity differences, the crystal structure of 8 was also determined. A comparison of the structures of planar Ni(II) complexes containing cis-dithiolate ligands, strongly suggests that the differences in reactivity are determined in part by the degree of flexibility that is allowed by the NN' chelate ring.
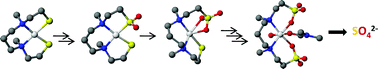

 Please wait while we load your content...
Please wait while we load your content...