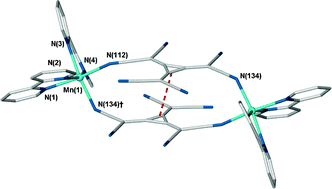
Several new first-row transition-metal complexes have been synthesised by combining the polynitrile dianion HCTMCP2− (hexacyanotrimethylenecyclopropandiide) with neutral, chelating co-ligands; 2,2′-bipyridine, 1,10-phenanthroline and 3-(2-pyridyl)pyrazole. The products cover a remarkable range of species including mononuclear complexes, dimers, charge-separated species and coordination polymers. Complexes containing 2,2′-bipyridine take the form [Mn(2,2′-bipy)2(HCTMCP)]2·2MeOH (1) or [M(2,2′-bipy)3](HCTMCP) (2Fe and 2Co) which are dimeric and charge-separated products, respectively. The products obtained using 1,10-phenanthroline were the discrete complex [Co(HCTMCP)(1,10-phen)2(H2O)]·H2O·MeCN (3) and the 1D coordination polymer [Mn(HCTMCP)(1,10-phen)(H2O)(MeOH)] (4). Complexes using the 3-(2-pyridyl)pyrazole co-ligand (pypzH) form similar 1D complexes to 4, namely [Mn(pypzH)(HCTMCP)(MeOH)(H2O)] (5) and [M(pypzH)(HCTMCP)(MeOH)2] (6Co and 6Fe), albeit with different hydrogen-bonding motifs between the chains. The polymeric HCTMCP complexes show weak to zero antiferromagnetic coupling between metal centres and thus no long-range ordering.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...