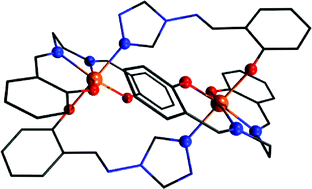
Four new iron(III) complexes were obtained by the reaction of 4-salicylideneamino-1,2,4-triazole (Hsaltrz) and selected dinuclear μ-oxo-bridged iron(III) Schiff base complexes [{FeL4}2(μ-O)], where L4 represents a terminal tetradentate dianionic Schiff-base ligand. X-ray structural analysis revealed a novel bridging mode of κN,κO of the saltrz ligand to form dinuclear complexes [{Fe(salen)(μ-saltrz)}2]·CH3OH (1) (H2salen = N,N′-ethylenebis(salicylimine)) and [{Fe(salpn)(μ-saltrz)}2] (2) (H2salpn = N,N′-1,2-propylenbis(salicylimine)), whereas one-dimensional (1D) zig-zag chains were formed in the case of [{Fe(salch)(μ-saltrz)}·0.5CH3OH]n (3) (H2salch = N,N′-cyclohexanebis(salicylimine)) and [Fe(salophen)(μ-saltrz)]n (4) (H2salophen = N,N′-o-phenylenebis(salicylimine)). It was also shown that the rigidity of the terminal ligand L4 can be considered as the key factor for the molecular dimensionality of the products. The thorough magnetic analysis based on SQUID experiments, including the isotropic exchange and the zero-field splitting of both temperature and field dependent data, was performed for dimeric (1 and 2) and also for polymeric compounds (3 and 4) and revealed weak antiferromagnetic exchange mediated by the saltrz anions with much larger D-parameter (|D| ≫ |J|).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...