Synthesis and structure of cationic guanidinate-bridged bimetallic {Li7M} cubes (M = Mn, Co, Zn) with inverse crown counter anions†
Abstract
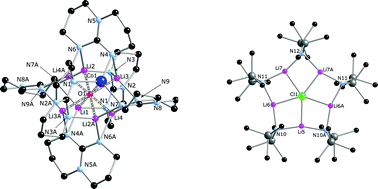
The reactions of the heteroleptic lithium amide [Li3(μ-hmds)2(μ,μ-hpp)] (1), where [hmds]− = hexamethyldisilazide and [hpp]− = hexahydropyrimidopyrimidide, with MnCl2, CoCl2 or ZnBr2 result in the formation of the separated ion-pairs [MLi7(μ8-O)(μ,μ-hpp)6]+[A]−, which each consist of a {MLi7} oxo-centred cube structural motif (M = Mn 2, Co 4, Zn 5), with each face of the cube being bridged by an [hpp]−

 Please wait while we load your content...
Please wait while we load your content...