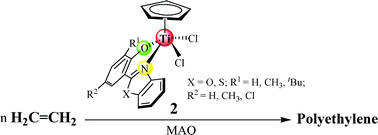
Half-sandwich titanium salicylbenzoxazole complexes CpTiLCl22a–2c [L = R-2-(benzo[d]xazol-2-yl)phenol (R = H (2a), R = 6-CH3 (2b), R = 4-CH3-6-tBu (2c)] and salicylbenzothiazole complexes CpTiLCl22d–2g [L = R-2-(benzo[d]thiazol-2-yl)phenol (R = H (2d), R = 6-CH3 (2e), R = 6-tBu (2f), R = 4-Cl (2g)] were synthesized by the reaction of CpTiCl3 with the sodium salts of their corresponding precursors. Complexes 2a–2g were fully characterized by 1H and 13C NMR spectra and elemental analyses. The molecular structures of 2a and 2b were determined by single crystal X-ray diffraction methods. When activated by excess methylaluminoxane (MAO) these half-sandwich titanium complexes showed moderate to high activities for ethylene polymerization and produced high molecular weight polyethylenes. The half-sandwich titanium salicylbenzoxazole complexes (2a–2c) exhibited higher activities, of up to 1.23 × 106 g PE mol Ti−1 h−1 for the 2b/MAO system, than those of their analogues, half-sandwich titanium salicylbenzothiazole complexes (2d–2g).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...