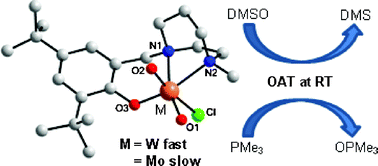
The synthesis and characterization of a series of molybdenum ([MoO2Cl(Ln)]; L1 (1), L2 (3)) and tungsten ([WO2Cl(Ln)]; L1 (2), L2 (4)) dioxo complexes (L1 = 1-methyl-4-(2-hydroxybenzyl)-1,4-diazepane and L2 = 1-methyl-4-(2-hydroxy-3,5-di-tert-butylbenzyl)-1,4-diazepane) of tridentate aminomonophenolate ligands HL1 and HL2 are reported. The ligands were obtained by reductive amination of 1-methyl-1,4-diazepane with the corresponding aldehyde. Complexes 3 and 4 were obtained by the reaction of [MO2Cl2(dme)n] (M = Mo, n = 0; W, n = 1) with the corresponding ligand in presence of a base, whereas for the preparation of 1 and 2 the ligands were deprotonated by KH prior to the addition to the metal. They were characterized by NMR and IR spectroscopy, by cyclic voltammetry, mass spectrometry, elemental analysis and by single-crystal X-ray diffraction analysis. Solid-state structures of the molybdenum and tungsten cis-dioxo complexes reveal hexa-coordinate metal centers surrounded by two oxo groups, a chloride ligand and by the tridentate monophenolate ligand which coordinates meridionally through its [ONN] donor set. In the series of compounds 1–4, complexes 3 and 4 have been used as catalysts for the oxygen atom transfer reaction between dimethyl sulfoxide (DMSO) and trimethyl phosphine (PMe3). Surprisingly, faster oxygen atom transfer (OAT) reactivity has been observed for the tungsten complex [WO2Cl(L2)] (4) in comparison to its molybdenum analog [MoO2Cl(L2)] (3) at room temperature. The kinetic results are discussed and compared in terms of their reactivity.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...