Synthesis and hydroamination catalysis with 3-aryl substituted pyrrolyl and dipyrrolylmethane titanium(iv) complexes†
Abstract
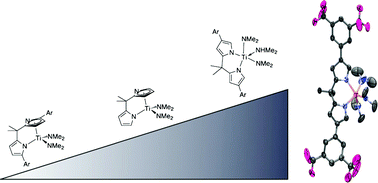
Using an iridium-catalyzed borylation/Suzuki–Miyaura coupling sequence several 3-aryl-pyrroles were accessed; in addition, dipyrrolylmethanes incorporating these 3-arylpyrroles were synthesized. Using the monodentate pyrrolyls, Ti(NMe2)2(HNMe2)(pyr3,5-CF3)2 (1) and a 2,4-diarylpyrrolyl complex Ti(NMe2)3(pyrAr/Ar′) (2) were prepared and structurally characterized. Titanium species bearing the new dipyrrolylmethane
- This article is part of the themed collection: d0 organometallics in catalysis

 Please wait while we load your content...
Please wait while we load your content...