*
Corresponding authors
a
Centre for Radiochemistry Research, School of Chemistry, The University of Manchester, Oxford Road, Manchester, United Kingdom
E-mail:
clint.a.sharrad@manchester.ac.uk
Fax: +44 161 275 4598
Tel: +44 161 275 4657
b
School of Chemistry, The University of Manchester, Oxford Road, Manchester, United Kingdom
c
School of Chemistry, Main Building, Cardiff University, Park Place, Cardiff, United Kingdom
d
School of Chemistry, Manchester Interdisciplinary Biocentre, The University of Manchester, 131 Princess Street, Manchester, United Kingdom
e
School of Chemical Engineering and Analytical Science, The University of Manchester, Oxford Road, Manchester, United Kingdom
f
Research Centre for Radwaste and Decommissioning, Dalton Nuclear Institute, The University of Manchester, Oxford Road, Manchester, United Kingdom
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) U
U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch. This stretching mode is also observed in the infrared spectra for all complexes 1–5 (818–826 cm−1) predominantly caused by the distortion of the tetradentate (salmnt(Et2N)2)2−
O stretch. This stretching mode is also observed in the infrared spectra for all complexes 1–5 (818–826 cm−1) predominantly caused by the distortion of the tetradentate (salmnt(Et2N)2)2− 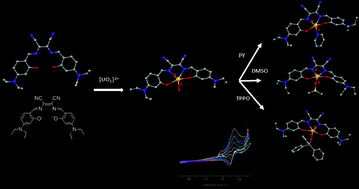

 Please wait while we load your content...
Please wait while we load your content...