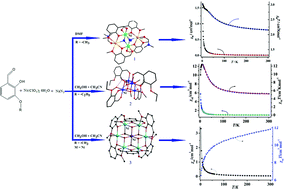
Three polynuclear complexes, [NiNa(μ1,1,1-N3)(μ-hmb)2(DMF)]2, (1), [Ni4(μ3-OMe)4(heb)4(MeOH)1.05(H2O)2.95], (2) and [NiIII(OH)6(hmb)6NiII6]·(ClO4)3 (3) (Hhmb = 2-hydroxy-3-methoxy-benzaldehyde; Hheb = 2-hydroxy-3-ethoxy-benzaldehyde), were prepared by reaction of the appropriate ligand with nickel(II) perchloride hexahydrate under solvothermal conditions. All compounds were characterized by elemental analysis, IR spectroscopy and X-ray single-crystal diffraction. Compound 1 exhibits a centrosymmetric heterotetranuclear cluster which represents the first nickel complex to possess two connected face-sharing cubes structure {Ni2Na2N2O4}. Compound 2 has a tetranuclear Ni cluster with a cubane topology in which the Ni(II) and the oxygen atoms from the methanol ligands occupying alternate vertices of the cube. Compound 3 consisits of a mixed-valence [NiIII(OH)6(hmb)6NiII6]3+ subunits and it represents the first nickel {NiII6NiIII} complex to possess a planar hexagonal disc-like structure. The results show that the minor ligand modifications or solvent change have a key role in the structural control of the self-assembly process. Magnetic properties of 1–3 in the 300–2 K have been discussed. The {Ni2Na2} (1) and {Ni4} (2) core display dominant ferromagnetic interactions from the nature of the binding modes through μ3-N3− or μ3-OCH3−, while {NiII6NiIII} core (3) displays dominant anti-ferromagnetic interactions from the nature of the binding modes through μ3-OH−.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...