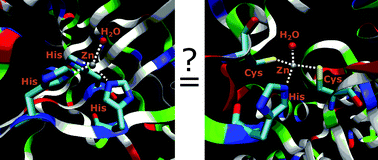
Comparison between α- and β-carbonic anhydrases: can Zn(His)3(H2O) and Zn(His)(Cys)2(H2O) sites lead to equivalent enzymes?†
Abstract
Large models of α- and β-carbonic anhydrases were compared using DFT calculations. They indicate similar acidity of the coordinated

 Please wait while we load your content...
Please wait while we load your content...