New binuclear MnII and FeII complexes supported by 1,4,8-triazacycloundecane†
Abstract
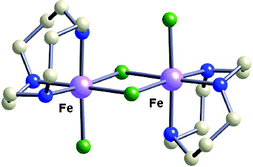
Two new binuclear metal complexes supported by ![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. For 1, a = 7.7321(12) Å, b = 7.8896(12) Å, c = 11.4945(17) Å, α = 107.832(2)°, β = 107.827(2)°, γ = 92.642(2)°, V = 627.85(17) Å3 and Z = 1. For 2, a = 7.7607(12) Å, b = 7.9068(12) Å, c = 11.6111(18) Å, α = 108.201(2)°, β = 108.041(2)°, γ = 92.118(3)°, V = 636.47(17) Å3 and Z = 1. Variable-temperature and variable-field magnetic susceptibility studies on 1 indicate the presence of weak ferromagnetic interactions between the high-spin iron(II) centers in the dimer (J = + 1.6 cm−1) and the crystalline field anisotropy of the ferrous ion (D = − 2.8, E = − 0.1 cm−1). Variable temperature magnetic susceptometry studies on 2 indicate that weak antiferromagnetic coupling exists between the manganese(II) centers (J = − 1.8 cm−1). Compounds 1 and 2 retain their dinuclearity in weakly coordinating or low polarity
space group. For 1, a = 7.7321(12) Å, b = 7.8896(12) Å, c = 11.4945(17) Å, α = 107.832(2)°, β = 107.827(2)°, γ = 92.642(2)°, V = 627.85(17) Å3 and Z = 1. For 2, a = 7.7607(12) Å, b = 7.9068(12) Å, c = 11.6111(18) Å, α = 108.201(2)°, β = 108.041(2)°, γ = 92.118(3)°, V = 636.47(17) Å3 and Z = 1. Variable-temperature and variable-field magnetic susceptibility studies on 1 indicate the presence of weak ferromagnetic interactions between the high-spin iron(II) centers in the dimer (J = + 1.6 cm−1) and the crystalline field anisotropy of the ferrous ion (D = − 2.8, E = − 0.1 cm−1). Variable temperature magnetic susceptometry studies on 2 indicate that weak antiferromagnetic coupling exists between the manganese(II) centers (J = − 1.8 cm−1). Compounds 1 and 2 retain their dinuclearity in weakly coordinating or low polarity

 Please wait while we load your content...
Please wait while we load your content...