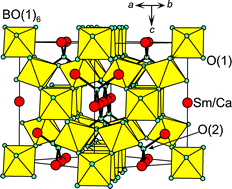
Proton conduction in three pyrochlores, Sm1.92Ca0.08B2O7−δ, B = Ti, Sn, Zr and one phase with a related C-type fluorite superstructure, B = Ce, has been investigated. The samples were prepared by solid state reaction. Infrared spectroscopy measurements and thermogravimetric analysis were carried out to study the extent of proton dissolution and determine its dependence on the B-site ion. Electrochemical impedance spectroscopy, performed on heating and cooling pre-hydrated samples, confirmed significant levels of proton conduction for Sm1.92Ca0.08Ti2O7−δ and Sm1.92Ca0.08Sn2O7−δ up to T ∼ 500 °C. In comparison the B = Zr and Ce samples revealed lower levels of proton conductivity, confined to temperatures below ∼ 400 °C. Proton diffusion coefficients of 3.36 × 10−8, 1.73 × 10−9, 5.53 × 10−10 and 2.78 × 10−11 cm2s−1 were determined at 300 °C for samples with B = Ti, Sn, Zr and Ce respectively. The proton mobility of Sm1.92Ca0.08Ti2O7−δ is therefore approximately one order of magnitude lower than that found in yttrium-doped perovskite phases such as BaZrO3 and BaCeO3.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...