A series of N1,N1,N3-tri-substituted benzamidrazones of the general formula [PhC(NHR)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NNMe2] (R = Me, n-Pr, i-Pr, n-Bu, Bn, Ph; 1a–f) was synthesized via condensation of 1,1-dimethylhydrazine with the corresponding imidoyl chloride, [PhC(Cl)
NNMe2] (R = Me, n-Pr, i-Pr, n-Bu, Bn, Ph; 1a–f) was synthesized via condensation of 1,1-dimethylhydrazine with the corresponding imidoyl chloride, [PhC(Cl)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NR]. Multinuclear NMR data, and zero-point energy DFT calculations conducted with the B3LYP functional and 6–31G+(d,p) basis set, suggest that these compounds exist as a single tautomer in solution; possessing a weak intramolecular hydrogen bond and a structure dominated by the localised resonance structure ArC(NHR)
NR]. Multinuclear NMR data, and zero-point energy DFT calculations conducted with the B3LYP functional and 6–31G+(d,p) basis set, suggest that these compounds exist as a single tautomer in solution; possessing a weak intramolecular hydrogen bond and a structure dominated by the localised resonance structure ArC(NHR)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–NMe2. An X-ray crystallographic study upon PhC(NHPh)
N–NMe2. An X-ray crystallographic study upon PhC(NHPh)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NNMe2 (1f) demonstrated that this compound adopts an identical tautomer in the solid state. Reactions of [PhC(NHMe)
NNMe2 (1f) demonstrated that this compound adopts an identical tautomer in the solid state. Reactions of [PhC(NHMe)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NNMe2] (1a) with [LMCl2]2 (M = Ru, L = cymene; M = Rh, Ir, L = Cp*) results in the stoichiometric formation of products of the formula [LM{PhC(
NNMe2] (1a) with [LMCl2]2 (M = Ru, L = cymene; M = Rh, Ir, L = Cp*) results in the stoichiometric formation of products of the formula [LM{PhC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NMe)NHNMe2}Cl]+Cl− (2a–c) in which the amidrazone chelates the metal in a κ2-N1,N3-coordination mode. Formation of this five-membered chelate occurs with a concomitant tautomerisation of the amidrazone ligand to an alternative tautomer, i.e. [PhC(
NMe)NHNMe2}Cl]+Cl− (2a–c) in which the amidrazone chelates the metal in a κ2-N1,N3-coordination mode. Formation of this five-membered chelate occurs with a concomitant tautomerisation of the amidrazone ligand to an alternative tautomer, i.e. [PhC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NMe)NHNMe2], the latter tautomer is expected to be readily energetically accessible based upon the aforementioned DFT calculations. This series of salts may be deprotonated with lithium hexamethyldisilazide to form the corresponding charge neutral complexes [LM{PhC(NMe)
NMe)NHNMe2], the latter tautomer is expected to be readily energetically accessible based upon the aforementioned DFT calculations. This series of salts may be deprotonated with lithium hexamethyldisilazide to form the corresponding charge neutral complexes [LM{PhC(NMe)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NNMe2}] (3a–c). In contrast, the reaction of N1,N1,N3-tri-substituted benzamidrazones with [(cymene)RuCl2]2 in the presence of NaOAc yielded a mixture of cyclometallation (C–H activation) and amidrazone chelation/deprotonation (N-H activation) products. Reaction of 1a yielded an inseparable mixture of products, whilst the reaction of 1c resulted in formation of the cyclometallated product [LM{C6H5C(
NNMe2}] (3a–c). In contrast, the reaction of N1,N1,N3-tri-substituted benzamidrazones with [(cymene)RuCl2]2 in the presence of NaOAc yielded a mixture of cyclometallation (C–H activation) and amidrazone chelation/deprotonation (N-H activation) products. Reaction of 1a yielded an inseparable mixture of products, whilst the reaction of 1c resulted in formation of the cyclometallated product [LM{C6H5C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NiPr)NHNMe2}] (L = cymene, M = Ru; 4a) in a modest 62% yield. This latter complex could be isolated as a crystalline orange solid, full characterisation including single crystal X-ray diffraction demonstrated that the amidrazone coordinates in a κ2-N2,C-coordination mode.
NiPr)NHNMe2}] (L = cymene, M = Ru; 4a) in a modest 62% yield. This latter complex could be isolated as a crystalline orange solid, full characterisation including single crystal X-ray diffraction demonstrated that the amidrazone coordinates in a κ2-N2,C-coordination mode.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NNMe2] (R = Me,
NNMe2] (R = Me, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NR]. Multinuclear
NR]. Multinuclear ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–NMe2. An X-ray crystallographic study upon PhC(NHPh)
N–NMe2. An X-ray crystallographic study upon PhC(NHPh)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NNMe2 (1f) demonstrated that this compound adopts an identical tautomer in the solid state. Reactions of [PhC(NHMe)
NNMe2 (1f) demonstrated that this compound adopts an identical tautomer in the solid state. Reactions of [PhC(NHMe)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NNMe2] (1a) with
NNMe2] (1a) with ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NMe)NHNMe2}Cl]+
NMe)NHNMe2}Cl]+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NMe)NHNMe2], the latter tautomer is expected to be readily energetically accessible based upon the aforementioned DFT calculations. This series of salts may be deprotonated with
NMe)NHNMe2], the latter tautomer is expected to be readily energetically accessible based upon the aforementioned DFT calculations. This series of salts may be deprotonated with ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NNMe2}] (3a–c). In contrast, the reaction of
NNMe2}] (3a–c). In contrast, the reaction of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NiPr)NHNMe2}] (L =
NiPr)NHNMe2}] (L = 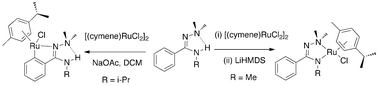

 Please wait while we load your content...
Please wait while we load your content...